Ionic bond

Ionic bonding involves a transfer of an electron, so one atom gains an electron while one atom loses an electron. One of the resulting ions carries a negative charge (anion), and the other ion carries a positive charge (cation). Because opposite charges attract, the atoms bond together to form a molecule.
Covalent bond

The most common bond in organic molecules, a covalent bond involves the sharing of electrons between two atoms. The pair of shared electrons forms a new orbit that extends around the nuclei of both atoms, producing a molecule. There are two secondary types of covalent bonds that are relevant to biology — polar bonds and hydrogen bonds.
Polar bond
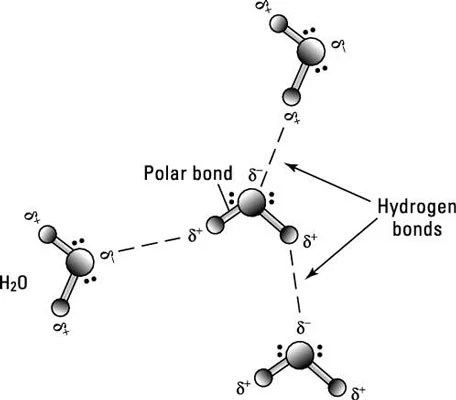
Two atoms connected by a covalent bond may exert different attractions for the electrons in the bond, producing an unevenly distributed charge. The result is known as a polar bond, an intermediate case between ionic and covalent bonding, with one end of the molecule slightly negatively charged and the other end slightly positively charged.
These slight imbalances in charge distribution are indicated in the figure by lowercase delta symbols with a charge superscript (+ or –). Although the resulting molecule is neutral, at close distances the uneven charge distribution can be important. Water is an example of a polar molecule; the oxygen end has a slight negative charge whereas the hydrogen ends are slightly positive. Polarity explains why some substances dissolve readily in water and others do not.
Hydrogen bond
Because they’re polarized, two adjacent H2O (water) molecules can form a linkage known as a hydrogen bond, where the (electropositive) hydrogen atom of one H2O molecule is electrostatically attracted to the (electronegative) oxygen atom of an adjacent water molecule.
Consequently, molecules of water join together transiently in a hydrogen-bonded lattice. Hydrogen bonds have only about 1/20 the strength of a covalent bond, yet even this force is sufficient to affect the structure of water, producing many of its unique properties, such as high surface tension, specific heat, and heat of vaporization. Hydrogen bonds are important in many life processes, such as in replication and defining the shape of DNA molecules.
About This Article
This article is from the book:
About the book authors:
Erin Odya is an anatomy and physiology teacher at Carmel High School in Carmel, Indiana, one of Indiana’s top schools. Maggie Norris is a freelance science writer living in the San Francisco Bay Area.